Clinical Diagnostic Solutions Revenue, Growth & Competitor Profile
Last updated:
Company Awards
Big Fish

Brain Power (IP)

Company Profile & Annual Report for Clinical Diagnostic Solutions
Access the complete profile.Clinical Diagnostic Solutions Fast Facts
| Revenue | $100 - $500 million See Exact Annual Revenue |
|---|---|
| Employees | 10 - 100Exact Company Size |
| Primary Industry | 325412 Pharmaceutical Preparation Manufacturing |
| Address | 1800 Nw 65th Ave Plantation, FL 33313 |
- What is the company's size? (Annual sales and employees)
- Clinical Diagnostic Solutions's annual revenues are $100 - $500 million (see exact revenue data)
- What industry is the company in?
- Clinical Diagnostic Solutions is classified as operating in the Pharmaceutical Preparation Manufacturing industry, NAICS Code 325412.
Clinical Diagnostic Solutions Annual Revenue and Growth Rate
| Clinical Diagnostic Solutions | Revenue Est. ($ Million) | Growth Rate (%) | # Employees |
|---|---|---|---|
| 2023 | $100 - $500 million Details in Premium Report | ||
| 2022 | |||
| 2021 | |||
| 2020 | |||
| 2019 | |||
|
1-Year Growth Rate: 3-Year Growth Rate (CAGR): | 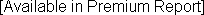 |
Note: Clinical Diagnostic Solutions's revenues are gauged from an analysis of company filings.
Clinical Diagnostic Solutions's Income Statement (based on Industry Averages)
| Clinical Diagnostic Solutions P&L | $ Millions |
|---|---|
| Revenue (Sales) | 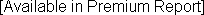 |
| Cost of Goods Sold | |
| Gross Profit | |
Operating Expenses | |
| Advertising | |
| Salaries and wages | |
| Other Operating Expenses | |
| Total Operating Expenses | |
| Operating Income | |
| EBITDA | |
| EBIT (Earnings Before Interest and Taxes) | |
| Net Profit | |
Trademark Applications
Trademark applications show the products and services that Clinical Diagnostic Solutions is developing and marketing. Clinical Diagnostic Solutions doesn't have any recent trademark applications, indicating Clinical Diagnostic Solutions is focusing on its existing business rather than expanding into new products and markets. Trademarks may include brand names, product names, logos and slogans.| Trademark | Date |
|---|---|
| CDS CLINICAL DIAGNOSTIC SOLUTIONS, INC. chemical reagents, lytic reagents, quench reagents, enzyme reagents, rinsing reagents and cleaning reagents all for use with hematology, and/or chemical analytical automated and semi-automated study and analysis instruments, machines and systems used in diagnostic, industrial, biological, and cytology laboratories all for scientific use in the human clinical laboratory testing market; reagents kits comprising reagents and buffer and standard solutions used in analytical chemistry packaged in ready-to-use dispensing packages for scientific use in the human clinical laboratory testing market; fluorescent dyes for scientific use, namely, for microscopic specimen slides and flow cytometry; Synthetic resin compounds in the form of microspheres used to incorporate other substances, and monodispersed test particles for use as standards, calibrators and carrier receptor sites employed with cytology and particle study and analysis instruments and systems used in the biological, industrial, and/or academic fields for scientific use in the human clinical laboratory testing market; calibrating and quality control fluid for blood analyzers, namely, whole blood hematology reference control solutions for calibration in electronic particle laboratory analysis for diagnostic, or scientific use in the human clinical testing laboratory testing market; Chemicals, namely, buffer and standard solutions used in analytical chemistry; Biochemical flow cytometry sheath fluids for scientific use; diagnostic laboratory reagents for hemoglobin testing for scientific use; reagents for use with automatic chemical analysis instruments in biological, chemical, industrial, and clinical laboratories for scientific use in the human clinical laboratory testing market | 03/08/2011 |
| NEXTGENERATION Chemical reagents, lytic reagents, quench reagents, enzyme reagents, rinsing reagents and cleaning reagents all for in vitro use with hematology, and/or chemical analytical automated and semi-automated study and analysis in the human clinical laboratory testing market; instruments, machines and systems used in diagnostic, industrial, biological, and cytology laboratories all for scientific use; Reagents kits comprising reagents and buffer and standard solutions used in analytical chemistry packaged in ready-to-use dispensing packages for scientific use; Fluorescent dyes for scientific use, namely, for microscopic specimen slides and flow cytometry; Synthetic resin compounds in the form of microspheres used to incorporate other substances, and monodispersed test particles for use as standards, calibrators and carrier receptor sites employed with cytology and particle study and analysis instruments and systems used in the biological, industrial, and/or academic fields for scientific or clinical use; Calibrating and quality control fluid for blood analyzers, namely, whole blood hematology reference control solutions for calibration in electronic particle laboratory analysis for diagnostic, scientific use; Chemicals, namely, buffer and standard solutions used in analytical chemistry; Biochemical flow cytometry sheath fluids for scientific use; diagnostic laboratory reagents for hemoglobin testing for scientific use; reagents for use with automatic chemical analysis instruments in biological, chemical, industrial, and clinical laboratories for scientific use | 03/08/2011 |
See all trademarks and details in the Full Report.
Market Share of Clinical Diagnostic Solutions's Largest Competitors
A competitive analysis shows these companies are in the same general field as Clinical Diagnostic Solutions, even though they may not compete head-to-head. These are the largest companies by revenue. However, they may not have the largest market share in this industry if they have diversified into other business lines. The "Competition" section of a business plan or investment memorandum would start by analyzing the information about these companies. Competitive advantage comes from offering better pricing or superior products/service.| Company | Headquarters | Revenue ($ MM) |
|---|---|---|
| MERIDIAN MEDICAL TECHNOLOGIES | Columbia, MD | 100 |
| SYNTRON BIORESEARCH | Carlsbad, CA | 55 |
| RLS USA | Lake Zurich, IL | 49 |
| ALORA PHARMACEUTICALS | Alpharetta, GA | 42 |
| LANTHEUS HOLDINGS | North Billerica, MA | 33 |
| BROADVIEW INVESTMENTS | Royston, GA | 32 |
| RESULTS RNA | Orem, UT | 29 |
| CIVICA | Lehi, UT | 28 |
| HARMONY BIOSCIENCES | Plymouth Meeting, PA | 24 |
Nearby Competitors
These companies are similar in business line and location to Clinical Diagnostic Solutions. While some companies compete with neighboring businesses for customers, other companies may compete to attract skilled employees.| Company | Headquarters | Revenue ($ MM) |
|---|---|---|
| VITAL PHARMACEUTICALS | Weston, FL | 100 |
| ARNET PHARMACEUTICAL | Davie, FL | 65 |
| BIOMATRIX SPECIALTY PHARMACY | Plantation, FL | 27 |
| UNIPHARMA | Tamarac, FL | 23 |
| CLINICAL DIAGNOSTIC SOLUTIONS | Plantation, FL | 15 |
| NEW VISION PHARMACEUTICALS | Tamarac, FL | 11 |
Future Competition: Clinical Diagnostic Solutions's Fastest Growing Competitors
These companies are in the same general field as Clinical Diagnostic Solutions and are rapidly expanding. Companies may grow organically or through acquisition. In some cases apparently high growth rates may be caused by data that weren't available in previous years.| Company | Revenue ($ MM) |
|---|---|
 | |

 My Watch List
My Watch List