ThermoGenesis Holdings Revenue, Growth & Competitor Profile
DBA CESCA THERAPEUTICS INC.



Note: Revenues for privately held companies are statistical evaluations.
ThermoGenesis Holdings's Annual Report & Profile shows critical firmographic facts:
Note: ThermoGenesis Holdings's revenues are gauged from an analysis of company filings.
See all trademarks and details in the Full Report.
Last updated:
Company Awards
Public Company

Fast Growing

Brain Power (IP)

Company Profile & Annual Report for ThermoGenesis Holdings
Access the complete profile.ThermoGenesis Holdings Fast Facts
| Revenue | $10 - $100 million See Exact Annual Revenue |
|---|---|
| Employees | 10 - 100Exact Company Size |
| Primary Industry | 339112 Surgical & Medical Instrument Manufacturing |
| Additional NAICS Codes | 5417 Scientific Research & Development Services |
| Address | 2711 Citrus Road Rancho Cordova, CA 95742 |
- What is the company's size? (Annual sales and employees)
- ThermoGenesis Holdings's annual revenues are $10 - $100 million (see exact revenue data)
- What industry is the company in?
- ThermoGenesis Holdings is classified as operating in the Surgical & Medical Instrument Manufacturing industry, NAICS Code 339112.
ThermoGenesis Holdings Annual Revenue and Growth Rate
| ThermoGenesis Holdings | Revenue Est. ($ Million) | Growth Rate (%) | # Employees |
|---|---|---|---|
| 2023 | $10 - $100 million Details in Premium Report | ||
| 2022 | |||
| 2021 | |||
| 2020 | |||
| 2019 | |||
|
1-Year Growth Rate: 3-Year Growth Rate (CAGR): | 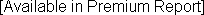 |
Note: ThermoGenesis Holdings's revenues are gauged from an analysis of company filings.
ThermoGenesis Holdings's Income Statement (based on Industry Averages)
| ThermoGenesis Holdings P&L | $ Millions |
|---|---|
| Revenue (Sales) | 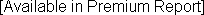 |
| Cost of Goods Sold | |
| Gross Profit | |
Operating Expenses | |
| Advertising | |
| Salaries and wages | |
| Other Operating Expenses | |
| Total Operating Expenses | |
| Operating Income | |
| EBITDA | |
| EBIT (Earnings Before Interest and Taxes) | |
| Net Profit | |
Trademark Applications
Trademark applications show the products and services that ThermoGenesis Holdings is developing and marketing. ThermoGenesis Holdings doesn't have any recent trademark applications, indicating ThermoGenesis Holdings is focusing on its existing business rather than expanding into new products and markets. Trademarks may include brand names, product names, logos and slogans.| Trademark | Date |
|---|---|
| COVID-IG medical diagnostic rapid test kits primarily comprising of blood collection needles, vials, test strips capable of visual or instrument read-out of test results, and/or vials of developer solution or reagents, which use finger stick or venipuncture whole blood, plasma, serum, and other bodily fluids and specimens for use in detecting antibodies, antigen, viruses, diseases, nucleic acids, pathogens and proteins; Diagnostic kits consisting primarily of monoclonal antibodies, buffers, and reagents for use in disease testing | 03/24/2020 |
| COVID-IGXPRESS Medical diagnostic rapid test kits primarily comprising of diagnostic test strips capable of visual or instrument read-out of test results and medical diagnostic reagents and developer solution for testing of bodily fluids and also including blood collection needles and vials, all for use in detecting antibodies, antigen, viruses, diseases, nucleic acids, pathogens and proteins; Diagnostic kits consisting primarily of monoclonal antibodies, buffers, and reagents for use in disease testing; all the above for use in relation to COVID-19 | 03/24/2020 |
| COVID-POCXPRESS Medical diagnostic rapid test kits primarily comprising of diagnostic test strips capable of visual or instrument read-out of test results and medical diagnostic reagents and developer solution for testing of bodily fluids and also including blood collection needles and vials, all for use in detecting antibodies, antigen, viruses, diseases, nucleic acids, pathogens and proteins; Diagnostic kits consisting primarily of monoclonal antibodies, buffers, and reagents for use in disease testing; all the above for use in relation to COVID-19 | 03/24/2020 |
See all trademarks and details in the Full Report.
Market Share of ThermoGenesis Holdings's Largest Competitors
A competitive analysis shows these companies are in the same general field as ThermoGenesis Holdings, even though they may not compete head-to-head. These are the largest companies by revenue. However, they may not have the largest market share in this industry if they have diversified into other business lines. The "Competition" section of a business plan or investment memorandum would start by analyzing the information about these companies. Competitive advantage comes from offering better pricing or superior products/service.| Company | Headquarters | Revenue ($ MM) |
|---|---|---|
| EMBECTA | Parsippany, NJ | 100 |
| STRUCTURE MEDICAL | Mooresville, NC | 34 |
| AUTOCAM MEDICAL DEVICES | Kentwood, MI | 30 |
| SCHARNBERG HOLDING | Des Moines, IA | 26 |
| SPECIALTY SURGICAL INSTRUMENTATION | Antioch, TN | 24 |
| MIDWEST INTERVENTIONAL SYSTEMS | Maple Grove, MN | 19 |
| VITA NEEDLE | Needham, MA | 17 |
| PYLANT MEDICAL | Grapevine, TX | 17 |
| MEDTEC | Orange City, IA | 16 |
Nearby Competitors
These companies are similar in business line and location to ThermoGenesis Holdings. While some companies compete with neighboring businesses for customers, other companies may compete to attract skilled employees.| Company | Headquarters | Revenue ($ MM) |
|---|---|---|
| BENTEC MEDICAL OPCO | Woodland, CA | 100 |
| CARTXPRESS BIO | Rancho Cordova, CA | 54 |
| THERMOGENESIS HOLDINGS | Rancho Cordova, CA | 49 |
| SERRA MEDICAL TRANSPORTATION | Elk Grove, CA | 48 |
| INNOBIOSURG OF AMERICA | El Dorado Hills, CA | 30 |
Future Competition: ThermoGenesis Holdings's Fastest Growing Competitors
These companies are in the same general field as ThermoGenesis Holdings and are rapidly expanding. Companies may grow organically or through acquisition. In some cases apparently high growth rates may be caused by data that weren't available in previous years.| Company | Revenue ($ MM) |
|---|---|
 | |

 My Watch List
My Watch List