Alliance Pharma Revenue, Growth & Competitor Profile
Last updated:
Company Awards
Fast Growing

Brain Power (IP)

Company Profile & Annual Report for Alliance Pharma
Access the complete profile.Alliance Pharma Fast Facts
| Revenue | $10 - $100 million See Exact Annual Revenue |
|---|---|
| Employees | 100 - 500Exact Company Size |
| Primary Industry | 5417 Scientific Research & Development Services |
| Additional NAICS Codes | 325412 Pharmaceutical Preparation Manufacturing 541711 Research & Development in Biotechnology 621511 Medical Laboratories |
| Address | 17 Lee Boulevard Malvern, PA 19355 |
- What is the company's size? (Annual sales and employees)
- Alliance Pharma's annual revenues are $10 - $100 million (see exact revenue data)
- What industry is the company in?
- Alliance Pharma is classified as operating in the Scientific Research & Development Services industry, NAICS Code 5417.
Alliance Pharma Annual Revenue and Growth Rate
| Alliance Pharma | Revenue Est. ($ Million) | Growth Rate (%) | # Employees |
|---|---|---|---|
| 2023 | $10 - $100 million Details in Premium Report | ||
| 2022 | |||
| 2021 | |||
| 2020 | |||
| 2019 | |||
|
1-Year Growth Rate: 3-Year Growth Rate (CAGR): | 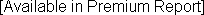 |
Note: Alliance Pharma's revenues are gauged from an analysis of company filings.
Alliance Pharma's Income Statement (based on Industry Averages)
| Alliance Pharma P&L | $ Millions |
|---|---|
| Revenue (Sales) | 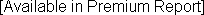 |
| Cost of Goods Sold | |
| Gross Profit | |
Operating Expenses | |
| Advertising | |
| Salaries and wages | |
| Other Operating Expenses | |
| Total Operating Expenses | |
| Operating Income | |
| EBITDA | |
| EBIT (Earnings Before Interest and Taxes) | |
| Net Profit | |
Trademark Applications
Trademark applications show the products and services that Alliance Pharma is developing and marketing. Alliance Pharma doesn't have any recent trademark applications, indicating Alliance Pharma is focusing on its existing business rather than expanding into new products and markets. Trademarks may include brand names, product names, logos and slogans.| Trademark | Date |
|---|---|
| RESOLIAN Laboratory testing services in clinical research and clinical trials of innovator and generic medicines in the pharmaceutical and biotechnology fields; Research and development in the fields of bioanalysis, immunoassay development, bioanalytical assay validation and testing, biomarker studies, drug metabolism, and pharmacokinetic testing; Pharmaceutical research and development services; Chemical, bioanalytical, biological and biotechnology research services; Research and development in the pharmaceutical and biotechnology fields; Research and development of vaccines and medicines; Pharmaceutical drug development services; Scientific testing in the fields of oncology, neurology, gastroenterology, immunology, endocrinology, gene therapy, infectious disease, rare and orphan diseases, cardiovascular disease, metabolic disease, central nervous system, ophthalmology, and respiratory diseases, for medical and pharmaceutical research purposes; Drug and chemical materials analysis services, namely, quality evaluation and analysis, quality assurance, and quality control, in the fields of pharmaceutical, biopharmaceutical, biotechnology and medical device development; Scientific analytical laboratory services; Good Manufacturing Practices (GMP) laboratory and stability testing and analytical services in the fields of pharmaceutical drug, pharmaceutical excipient, and consumer healthcare chemical development; Chemistry, Manufacturing, and Controls (CMC) laboratory and stability testing and analytical services in the fields of pharmaceutical drug, pharmaceutical excipient, and consumer healthcare chemical development; Analytical services, namely, providing Good Laboratory Practices (GLP) testing and quality assurance services to assess clinical performance in the field of therapeutic drug evaluation and development | 02/15/2023 |
| RESOLIAN BIOANALYTICS Laboratory testing services in clinical research and clinical trials of innovator and generic medicines in the pharmaceutical and biotechnology fields; Research and development in the fields of bioanalysis, immunoassay development, bioanalytical assay validation and testing, biomarker studies, drug metabolism, and pharmacokinetic testing; Pharmaceutical research and development services; Chemical, bioanalytical, biological and biotechnology research services; Research and development in the pharmaceutical and biotechnology fields; Research and development of vaccines and medicines; Pharmaceutical drug development services; Scientific testing in the fields of oncology, neurology, gastroenterology, immunology, endocrinology, gene therapy, infectious disease, rare and orphan diseases, cardiovascular disease, metabolic disease, central nervous system, ophthalmology, and respiratory diseases, for medical and pharmaceutical research purposes; Drug and chemical materials analysis services, namely, quality evaluation and analysis, quality assurance, and quality control, in the fields of pharmaceutical, biopharmaceutical, biotechnology and medical device development; Scientific analytical laboratory services; Good Manufacturing Practices (GMP) laboratory and stability testing and analytical services in the fields of pharmaceutical drug, pharmaceutical excipient, and consumer healthcare chemical development; Chemistry, Manufacturing, and Controls (CMC) laboratory and stability testing and analytical services in the fields of pharmaceutical drug, pharmaceutical excipient, and consumer healthcare chemical development; Analytical services, namely, providing Good Laboratory Practices (GLP) testing and quality assurance services to assess clinical performance in the field of therapeutic drug evaluation and development | 02/15/2023 |
| RESOLIAN ANALYTICAL SCIENCES Laboratory testing services in clinical research and clinical trials of innovator and generic medicines in the pharmaceutical and biotechnology fields; Research and development in the fields of bioanalysis, immunoassay development, bioanalytical assay validation and testing, biomarker studies, drug metabolism, and pharmacokinetic testing; Pharmaceutical research and development services; Chemical, bioanalytical, biological and biotechnology research services; Research and development in the pharmaceutical and biotechnology fields; Research and development of vaccines and medicines; Pharmaceutical drug development services; Scientific testing in the fields of oncology, neurology, gastroenterology, immunology, endocrinology, gene therapy, infectious disease, rare and orphan diseases, cardiovascular disease, metabolic disease, central nervous system, ophthalmology, and respiratory diseases, for medical and pharmaceutical research purposes; Drug and chemical materials analysis services, namely, quality evaluation and analysis, quality assurance, and quality control, in the fields of pharmaceutical, biopharmaceutical, biotechnology and medical device development; Scientific analytical laboratory services; Good Manufacturing Practices (GMP) laboratory and stability testing and analytical services in the fields of pharmaceutical drug, pharmaceutical excipient, and consumer healthcare chemical development; Chemistry, Manufacturing, and Controls (CMC) laboratory and stability testing and analytical services in the fields of pharmaceutical drug, pharmaceutical excipient, and consumer healthcare chemical development; Analytical services, namely, providing Good Laboratory Practices (GLP) testing and quality assurance services to assess clinical performance in the field of therapeutic drug evaluation and development | 02/15/2023 |
See all trademarks and details in the Full Report.
Market Share of Alliance Pharma's Largest Competitors
A competitive analysis shows these companies are in the same general field as Alliance Pharma, even though they may not compete head-to-head. These are the largest companies by revenue. However, they may not have the largest market share in this industry if they have diversified into other business lines. The "Competition" section of a business plan or investment memorandum would start by analyzing the information about these companies. Competitive advantage comes from offering better pricing or superior products/service.| Company | Headquarters | Revenue ($ MM) |
|---|---|---|
| LEIDOS | Denver, CO | 100 |
| NATIONAL TECHNOLOGY & ENGINEERING SOLUTIONS OF SANDIA | Albuquerque, NM | 30 |
| INC RESEARCH | Raleigh, NC | 27 |
| RESEARCH FOUNDATION FOR STATE UNIVERSITY OF NEW YORK | Albany, NY | 17 |
| FLUOR MARINE PROPULSION | West Mifflin, PA | 15 |
| SYNEOS HEALTH | Raleigh, NC | 13 |
| ILLUMINA | San Diego, CA | 12 |
| UT-BATTELLE | Oak Ridge, TN | 11 |
| PRA HOLDINGS | Raleigh, NC | 11 |
Nearby Competitors
These companies are similar in business line and location to Alliance Pharma. While some companies compete with neighboring businesses for customers, other companies may compete to attract skilled employees.| Company | Headquarters | Revenue ($ MM) |
|---|---|---|
| ENDO PHARMACEUTICALS | Malvern, PA | 100 |
| FRONTAGE LABORATORIES | Exton, PA | 73 |
| NEURONETICS | Malvern, PA | 21 |
| REACTION BIOLOGY | Malvern, PA | 16 |
| ALLIANCE PHARMA | Malvern, PA | 14 |
| OCUGEN | Malvern, PA | 10 |
Future Competition: Alliance Pharma's Fastest Growing Competitors
These companies are in the same general field as Alliance Pharma and are rapidly expanding. Companies may grow organically or through acquisition. In some cases apparently high growth rates may be caused by data that weren't available in previous years.| Company | Revenue ($ MM) |
|---|---|
 | |

 My Watch List
My Watch List